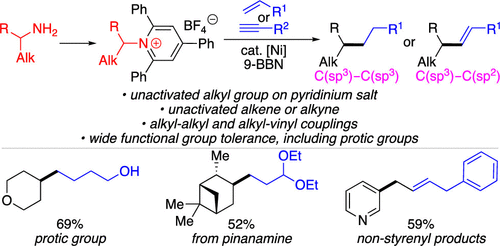
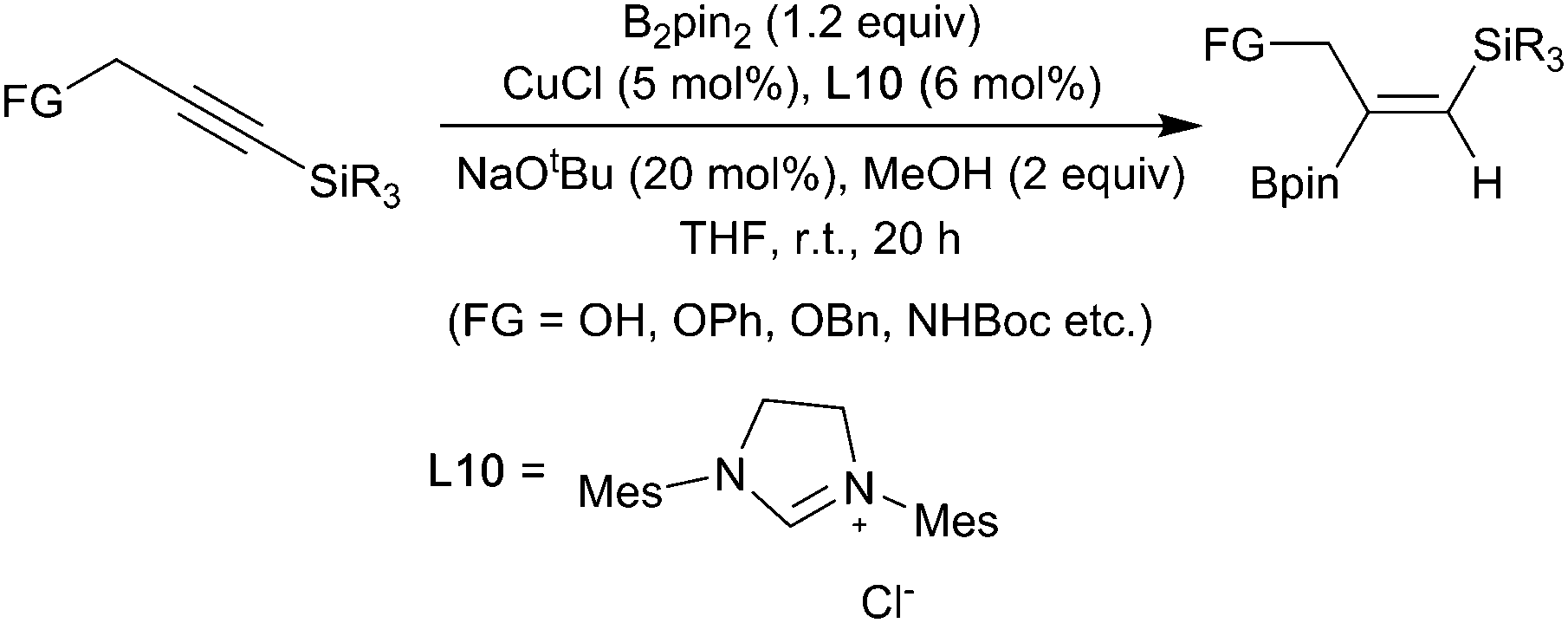
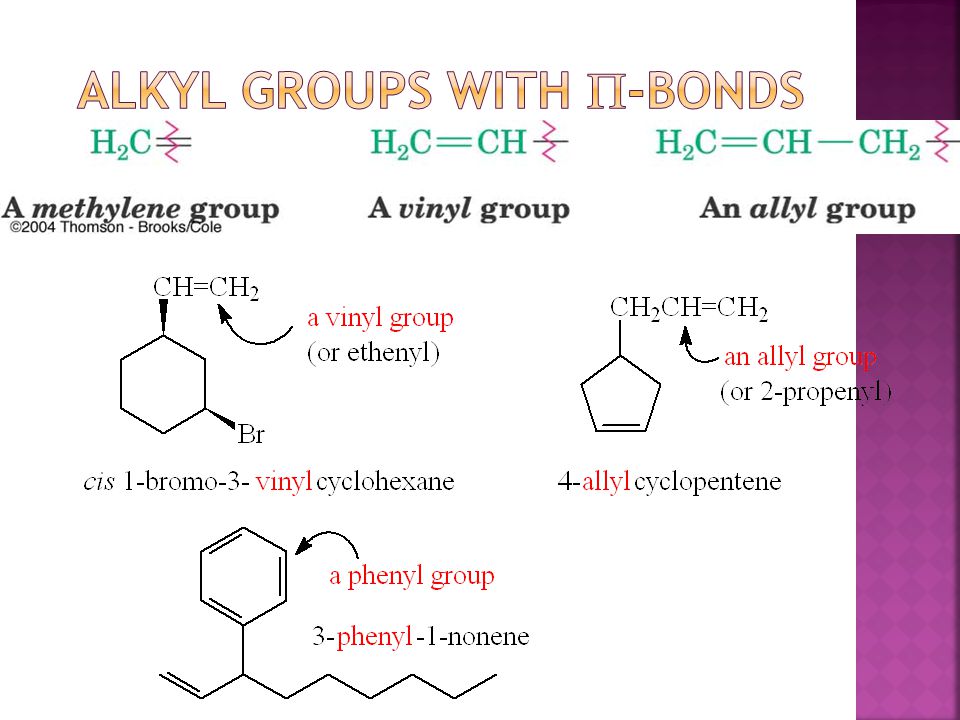The key to our success is the use of an earth abundant zirconium based catalyst which allows a balance of self contradictory reactivities dehydrogenative boration and hydroboration to be achieved.
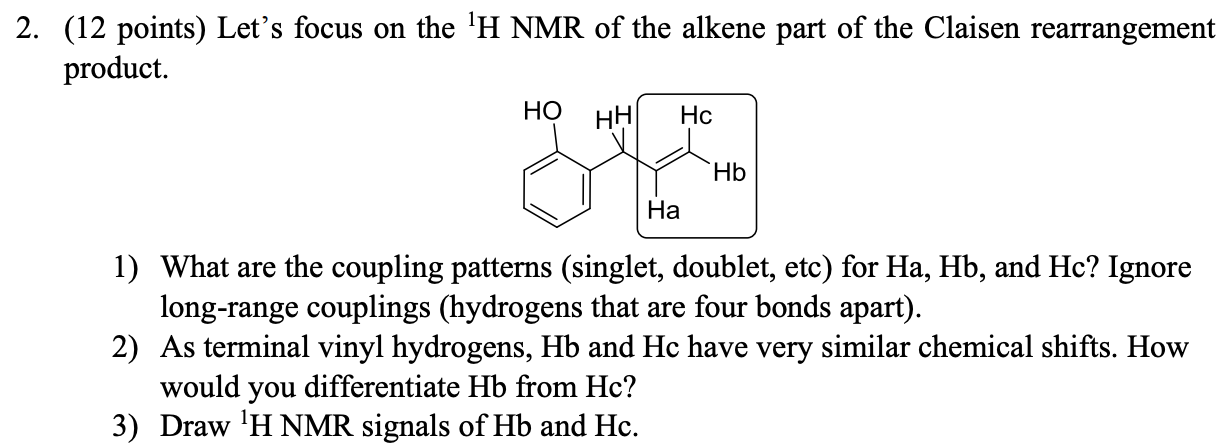
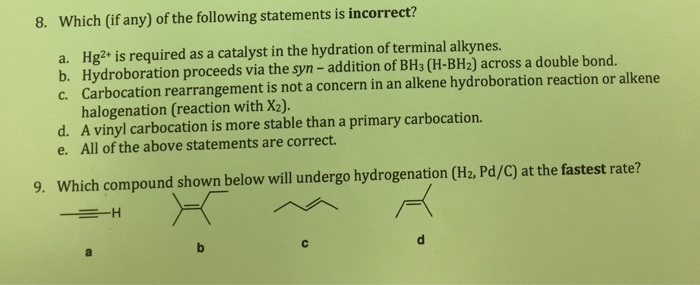
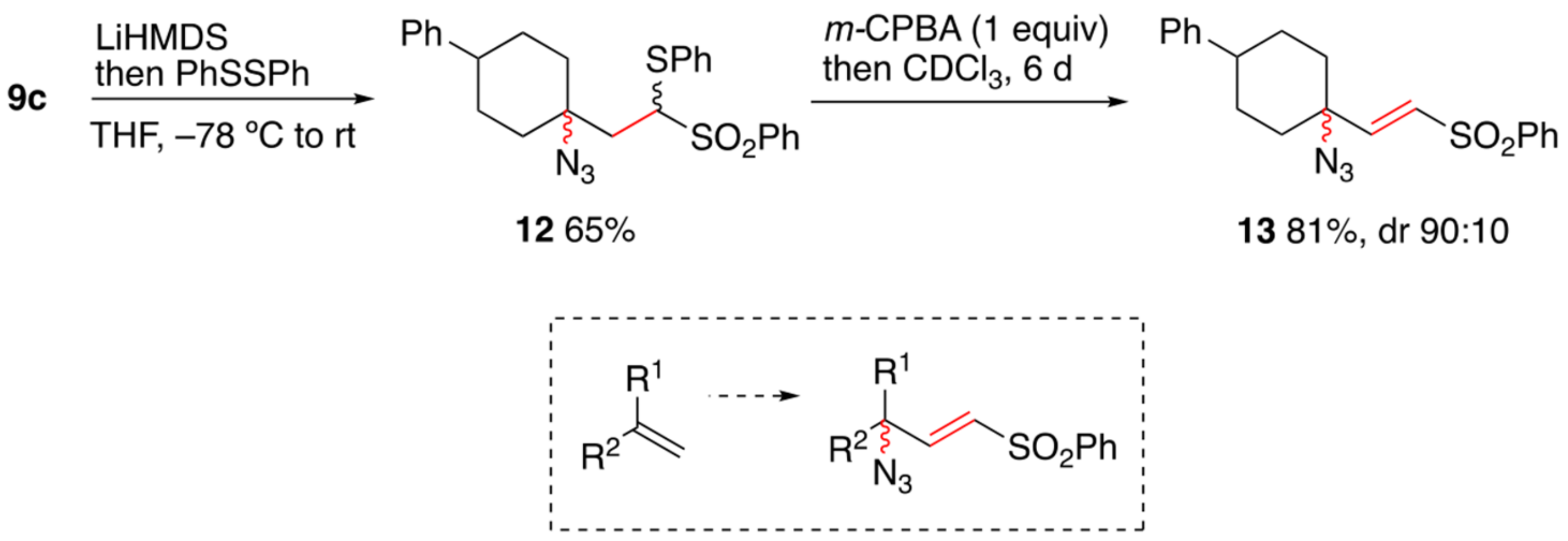
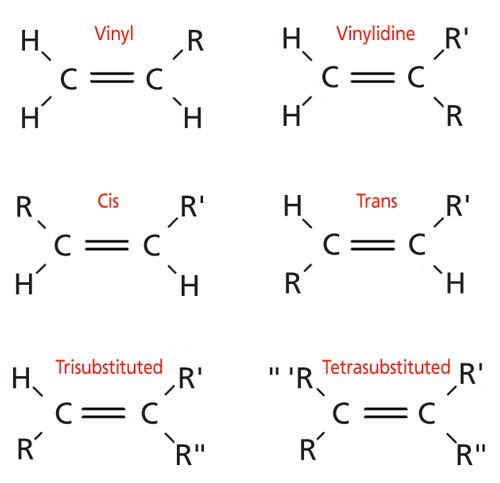
Terminal vinyl alkene.
1 methyl 2 methylsulfonyl benzimidazole reacts with a variety of aldehydes and ketones in the presence of either nahmds 55 c to rt or t buok rt 1 h in dmf to give the corresponding terminal alkenes in high yields the byproducts of this julia type methylenation reagent are easily removed and the reaction conditions are mild and practical.
Terminal vinyl groups can also be converted into alkenyl nucleophiles.
Again an internal alkene does not interfere.
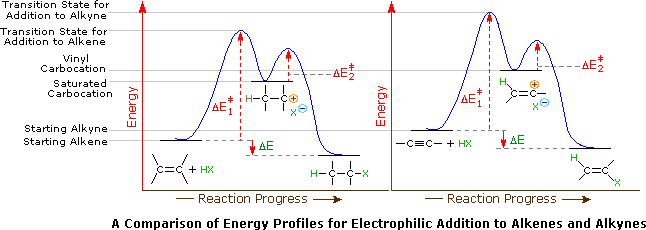
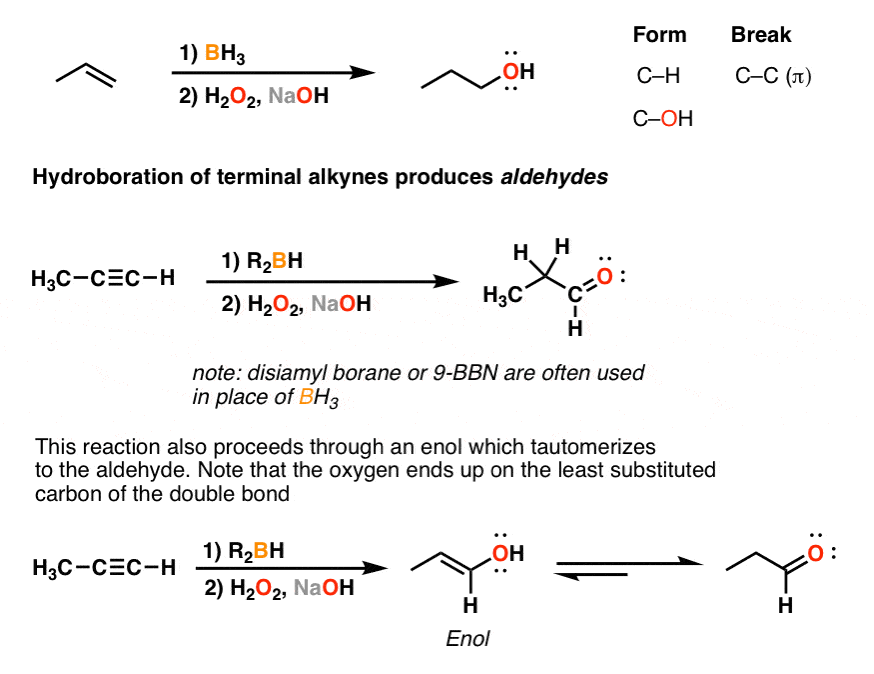
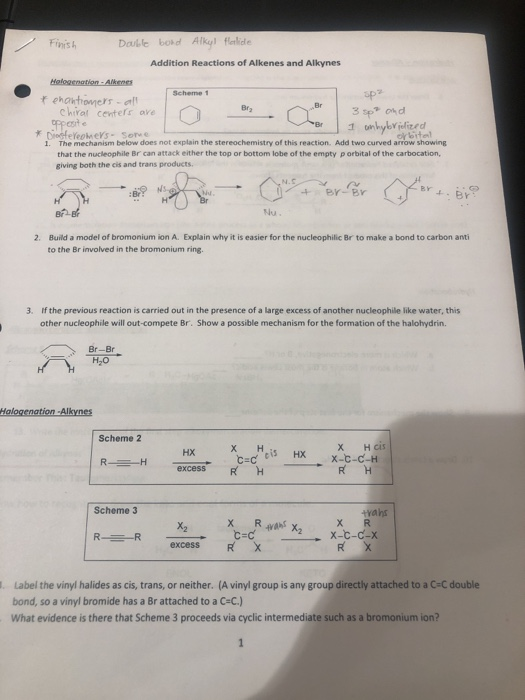
As with alkenes the b h reagent group adds in an apparently anti markovnikov manner due to the fact that the boron is the electrophile not the hydrogen.
For example the corresponding oxazolidinone phthalimide and acyclic acetamide 13 also react to deliver the desired products.
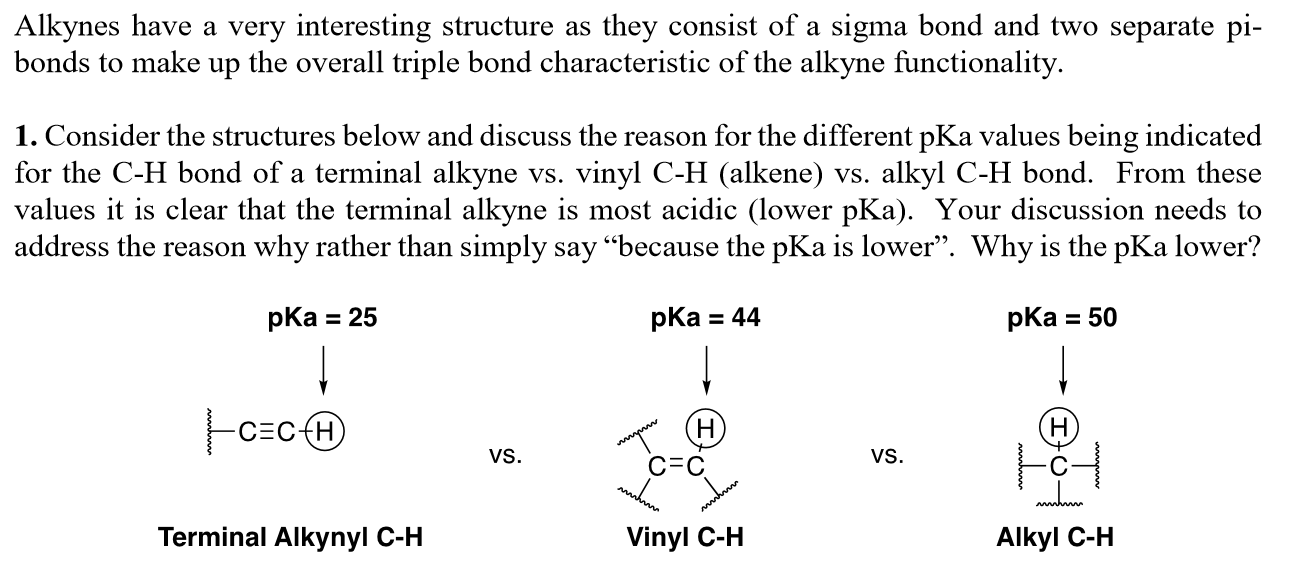
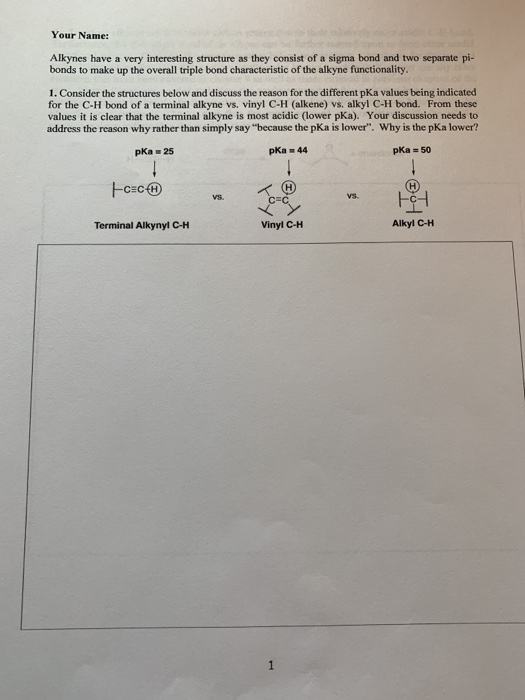
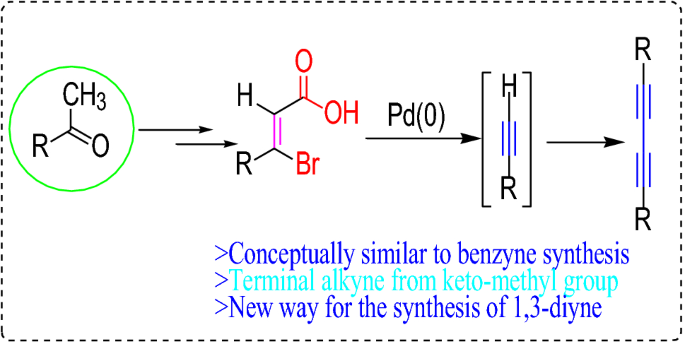
Terminal alkynes like acetylene itself are mildly acidic with pk a values of around 25.
The heck reaction of aryl bromide with a terminal alkene substrate having a chiral center at the allylic position and a phenyl substituent at another terminal carbon is reported.
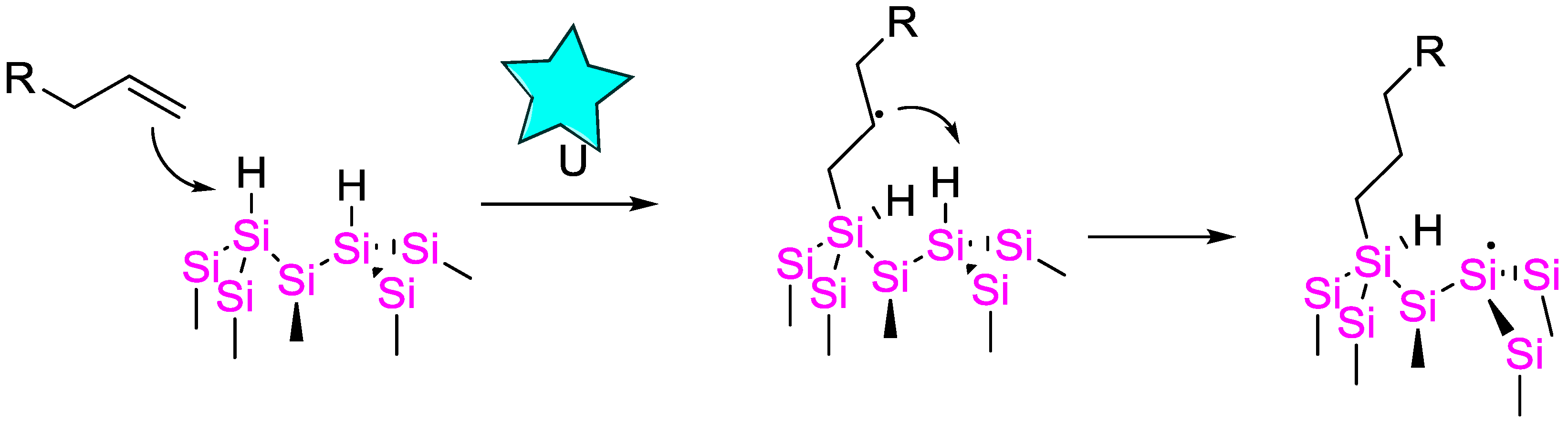
The authors performed radical clock experiments that provide evidence for the presence of radical species in related systems.
The acidic hydrogen on terminal alkynes can be replaced by a variety of groups resulting in halo silyl and alkoxoalkynes.
The name is also used for any compound containing that group namely r ch ch 2 where r is any other group of atoms.
Such as i affords two regioisomeric vinyl type alkenes.
The alkene scope is limited to terminal n vinyl pyrrolidinones.
A general and atom economical synthesis of 1 1 diborylalkanes from alkenes and a borane without the need for an additional h 2 acceptor is reported for the first time.
In chemistry vinyl or ethenyl abbreviated as vi is the functional group with the formula c h ch 2 it is the ethylene iupac ethene molecule h 2 c ch 2 with one fewer hydrogen atom.
And closely related substrates.
An industrially important example is vinyl chloride precursor to pvc a plastic.
An alkene migration to the phenyl substituted end carbon is observed along with the typical heck reaction.
Jamison of mit has shown j.
Further addition to the resulting boron substituted alkene does not occur and the usual oxidative.